- Original article
- Open access
- Published:
Magnesium sulphate within multimodal analgesia, pre-emptive, or preventive analgesia
Ain-Shams Journal of Anesthesiology volume 14, Article number: 7 (2022)
Abstract
Background
Magnesium (Mg) is a non-competitive N-methyl d-aspartate receptor antagonist with antinociceptive effects. Multimodal therapy is the optimal strategy for perioperative pain control to minimize the need for opioids. Inflammation caused by tissue trauma or direct nerve injury is responsible for the perioperative pain. The concept of “pre-emptive” analgesia, analgesic strategies administered prior to the stimulus, can modify the peripheral and central nervous system processing of noxious stimuli, thereby reducing central sensitization, hyperalgesia, and allodynia remains controversial. A more encompassing approach to the reduction of postoperative pain is the concept of “preventive” analgesia. The purpose of the study is to detect the proper use of MgSO4 as an analgesic being a non-competitive N-methyl d-aspartate (NMDA).
Results
There is no statistically significant difference in the haemodynamic parameters, intraoperative (33% vs 20%) and postoperative requirement for analgesics 6.6% vs 10% among groups I and II, respectively. There is no significant difference in the numerical analogue scale, where 16 vs 17 patients with no pain, 12 vs 10 with mild pain, and 2 vs 3 with moderate pain in groups I and II, respectively.
Conclusion
The use of MgSO4 in a bolus with or without infusion is comparable in the control of intraoperative and postoperative pain.
Backgrounds
Perioperative pain management is indicated to relieve the patients’ suffering, allow early mobilization after surgery, reduce the length of hospital stay, and have better satisfaction. Pain control regimens must consider medical, psychological, and physical conditions; age; level of fear or anxiety; surgical procedure; personal preference; and the patients’ response.
Traditionally, acute perioperative pain management is targeting the central mechanisms involved in the perception of pain by opioid medications (Mudumbai et al., 2016; Sun et al., 2016). Thus, opioid use has reached a critical level worldwide; accordingly, multimodal therapy is the optimal choice for perioperative pain control to minimize the need for opioids (Alam & Juurlink, 2016).
The perioperative pain is initiated either by an inflammatory process induced by tissue trauma or by direct nerve injury from nerve transection, stretching, or compression. Tissue trauma is not only initiating pain at the site of the trauma but also to the surrounding area through local inflammatory mediators that augment the sensitivity to stimuli (hyperalgesia) or even misperception of pain to non-noxious stimuli (allodynia). Other mechanisms contributing to hyperalgesia and allodynia include sensitization of the peripheral pain receptors (primary hyperalgesia) and increased excitability of central nervous system neurons (secondary hyperalgesia) (Kelly et al., 2001; Woolf & Chong, 1993a; Suzuki, 1995).
Multimodal analgesia is the analgesia achieved by using several agents instead of using a single agent, each acting at different sites of the pain pathway. This approach reduces the dependence on a single medication and reduces or eliminates the need for opioids. The Synergism between opioid and non-opioid medications reduces the required opioid dose and the side effects related to them.
Pain receptor activity can be blocked directly by (e.g. lidocaine) or indirectly by anti-inflammatory agents to diminish the local hormonal response to injury, thus decreasing the pain receptor activation.
Other analgesic agents (e.g. ketamine, gabapentin, pregabalin) modulate the activity of neurotransmitters (substance P, calcitonin gene-related peptide, aspartate, glutamate, and gamma-aminobutyric acid (GABA)), by inhibiting or augmenting their activity.
The concept of “pre-emptive” analgesia, meaning that analgesic strategies administered prior to surgical incision or stimulus can modify the peripheral and central nervous system processing of noxious stimuli, thereby reducing central sensitization, hyperalgesia, and allodynia (Kelly et al., 2001; Woolf & Chong, 1993a; Suzuki, 1995), remains controversial. Several studies have concluded that preoperative timing is not necessary to achieve a reduction in postoperative pain and opioid use (Møiniche et al., 2002a).
An approach with a wider spectrum to the reduction of acute as well as chronic postoperative pain is the concept of “preventive” analgesia. The aim of preventive analgesia is to reduce the sensitization to the perioperative noxious stimuli, by treatments administered at any time in the perioperative period (Rosero & Joshi, 2014; Katz et al., 2011).
Magnesium (Mg) is a non-competitive N-methyl d-aspartate (NMDA) receptor antagonist with analgesic effects (Mayer et al., 1984; McCarthy et al., 1998). It has been accepted as an adjuvant for intra- and postoperative analgesia. Perioperative magnesium sulphate reduces the need for anaesthetics and improves postoperative analgesia (Choi et al., 2002; Wilder-Smith et al., 1997). However, some claim that magnesium sulphate has limited if any effect does exist (Choi et al., 2002; Ko et al., 2001; Paech et al., 2006). The role of magnesium sulphate infusion on the consumption of anaesthetics and opioids has been reported to be variable depending on the procedures done (Schulz-Stubner et al., 2001; Telci et al., 2002).
However, since the magnesium ion poorly crosses the blood-brain barrier in humans, it is not clear whether the therapeutic effect is related to NMDA antagonism in the central nervous system, dorsal horn NMDA receptors, or peripheral (Buvanendran, 2011).
Owing to this “protective” effect on the nociceptive pathways, pre-emptive analgesia has the potential to be more effective than a similar analgesic treatment initiated after surgery(Dahl & Møiniche, 2004). Consequently, immediate postoperative pain may be reduced, and the development of chronic pain may be prevented (Woolf & Chong, 1993b).
Aim of work
The aim of the work is to detect the proper use of MgSO4 as an analgesic being a NMDA receptor blocker
Methods
The study is a blinded observational study that was conducted in Ain shams University Hospitals on 60 patients scheduled for a variety of surgical procedures. The study was approved by the Research Ethics Committee of the Faculty of Medicine, Ain Shams University and conducted in accordance with the principles of the Declaration of Helsinki. A written informed consent before enrolment was taken. The patients were randomly divided into two equal groups, group I and group II. Randomization was done by computer-generated number lists and used opaque sealed envelopes.
Sample size and statistics
The sample size was calculated using ClinCalc.com, setting the type 1 error (α) at 0.05, power (1 − β) at 0,.8 and confidence width level at 0.1. Calculation according to the values of similar studies produced a minimal sample size of 25 cases.
The Mann-Whitney test is used to compare non-parametric between the two study groups. The chi-square and Fisher’s exact tests were used to examine the relationship between categorical variables. P value < 0.05 was considered statistically significant. All statistical procedures were carried out using Microsoft Excel 365. The median and interquartile range were used for skewed numerical data, and percentage and proportions for categoric values.
The primary outcome is to study the proper timing for initiating the MgSo4 as an analgesic, and the secondary outcome is to study the complications of using MgSo4 as an analgesic.
Inclusion criteria
The following are the inclusion criteria:
-
Age 18–70 years
-
Both sex
-
ASA I, II, III, and IV
-
The patients scheduled for general anaesthesia
-
Procedure > 60 min with expected moderate to severe pain postoperative
Exclusion criteria
The following are the exclusion criteria:
-
Patients refusing to participate in the study
-
Patients with renal insufficiency
-
Patients with liver disease
A standard monitor was attached to the patients including 5 leads ECG, pulse oximeter, and NIBP, and the IV line was secured.
The anaesthesia was induced by propofol 1.5 mg/kg and atracurium 0.5 mg/kg, and the patients will be intubated by endotracheal tube size 7 for females and 8 for males; after then, the patients will be ventilated using volume-controlled mode at a rate of 4–6 ml/kg, RR 12 bpm
The patients in group I were given a MgSO4 50-mg/kg bolus dose with the induction of anaesthesia; the induction of anaesthesia was conducted with 100 μg of fentanyl, paracetamol 1 g and NSAID (Ketorolac) 30 mg/ml given during the procedure and 10 mg of nalbuphine by the end of the procedure. In group II, MgSO4 50 mg/kg bolus with the induction of anaesthesia and 100 μg of fentanyl were given in the induction phase. In addition to paracetamol 1 g and NSAID (Ketorolac) 30 mg/ml, MgSO4 at a dose of 15 mg/kg/hr were given during the procedure and 10 mg of nalbuphine by the end of the procedure.
When there was a change in the blood pressure and the heart rate by more than 20% of the preoperative value, 50 μg of fentanyl were given after excluding other possible causes.
Another incremental dose of fentanyl was given up to a total dose of 200 μg in addition to paracetamol on demand every 6 hr and NSAID (Ketorolac) 30 mg prn every 6 h up to a total of 120 mg per day during the procedure.
After extubating the patients, the pain scores were assessed after an hour using a numerical rating scale, where 0 = no pain and 10 = the worst pain that has ever been experienced. I classify the pain as from 0 to ≤ 3 as mild, 4 to less than 7 as moderate pain, and greater than or equal to 7 as severe pain. If it was recorded > 4, an additional 5 mg nalbuphine is given and the narcotics used were recorded.
In case of failure of control of the pain by these strategies, the patient was excluded and replaced by another.
The haemodynamic parameters recorded every 15 min including systolic BP, diastolic BP, and HR were collected. An average reading for the haemodynamic for each patient was recorded by the end of the procedure. The number of the patients who received intraoperative as well as postoperative adjuvant analgesics were recorded. The complications from using MgSo4 including hypotension, delayed recovery, visual changes, and respiratory paralysis were also monitored. The anaesthesia nurse who records the data and who was applying the NAS were blinded.
Results
The demographic data were comparable in both groups; most of the patients were ASA I and ASA II (Table 1).
There was no intraoperative statistically significant difference in the haemodynamic parameters among the two groups where the median for the systolic blood pressure was 120 mmHg in the two groups, 75 mmHg for the diastolic blood pressure among group I vs 70 mmHg among group II while the median for heart rate was 80 bpm vs 76 bpm in group II (Table 2).
There was no statistically significant increase in the requirement of intraoperative adjuvant and the postoperative requirement for analgesics 6.6% vs 10% with a P value < 0.05 among groups I and II, respectively (Table 3).
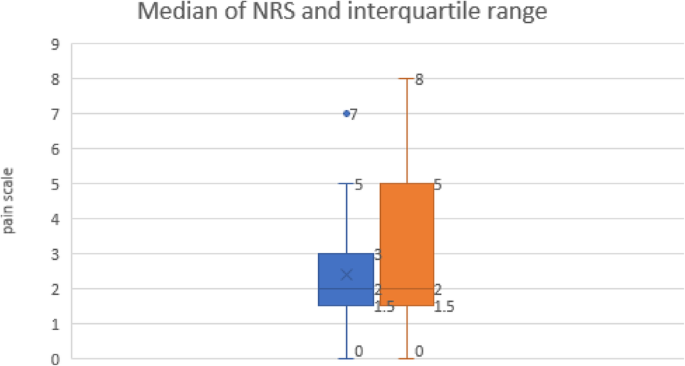
The numerical rating scale for pain was almost the same among the two groups with no significant statistical difference among them, where 16 patients in group I vs 17 in group II with no pain, 12 vs 10 with mild pain, 2 vs 3 with moderate pain, and no patients in any of the two groups complaining of severe pain [Table 4, Fig. 1]. The cliff’s delta statistics approach near to 0.0 (− 0.07) indicating that the compared groups tend to overlap, making the effect size correlate to non-statistical difference.
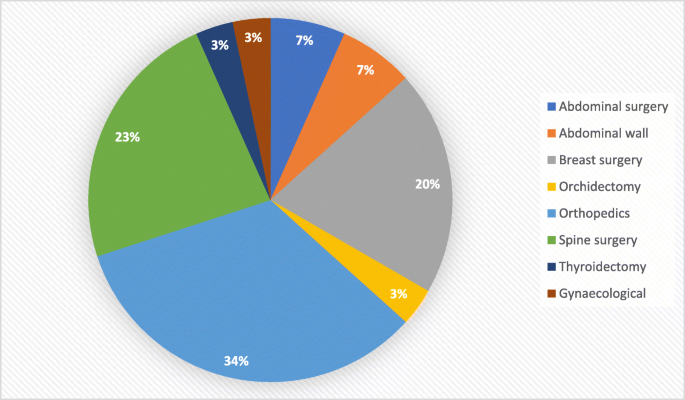
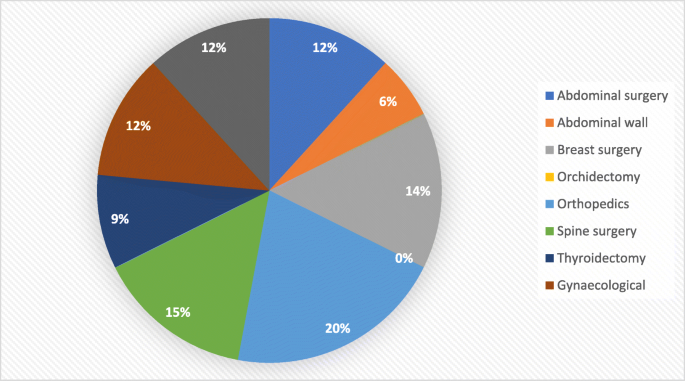
The types of surgery were similar in the two groups with the most frequent were abdominal surgery, spine, and orthopaedic surgeries [Figs. 2 and 3]. No reported complications were recorded in the two groups including hypotension, respiratory paralysis, delayed recovery, and abnormality in vision.
Discussion
Magnesium is a NMDA receptor antagonist. Intravenous magnesium has been accepted to be an effective adjuvant for the reduction of the opioid requirement, especially useful in opioid-tolerant patients or when there are medical concerns related to opioid dose.
In two meta-analyses trials, intraoperative IV magnesium sulphate has been proven to be superior compared with placebo in over 1200 patients in regard to reduced perioperative opioid consumption and pain scores in the first 24 h postoperatively, with no serious consequence (Mariano, 2020; Albrecht et al., 2013).
In one of the analyses, opioid consumption was dramatically decreased for morphine in 24 h, 24.4%, and the pain scores at 24 h after surgery were reduced to be 4.2 at rest and 9.2 on movement (De Oliveira Jr et al., 2013). Both bolus and continuous infusion regimens were effective.
Administration of magnesium at a dose of 40 mg/kg before induction, followed by a 10 mg/kg/h infusion, resulted in a decrease of the total opioid without any major haemodynamic consequences. Higher infusion doses have no added value (Buvanendran, 2011); however, Ryu et al. successfully uses a dose of 50 mg/kg magnesium sulphate intravenous as a bolus and then 15 mg/kg/hr by continuous intravenous infusion (Ryu et al., 2008).
The results of the current study match this analysis in the regard that there is neither difference in the requirement of analgesics nor the postoperative pain score between the bolus dose and the infusion; however, our study was carried out on a limited number of patients in early postoperative period.
Multimodal analgesia using magnesium may provide benefit especially when used with ketamine. In a trial of 50 patients scheduled for scoliosis surgery, the addition of magnesium to ketamine decreased postoperative morphine consumption by 30%, with improved sleep and satisfaction scores, but no change in pain scores (Jabbour et al., 2014).
The results from a lot of studies largely declare that the pre-emptive administration of analgesics in surgical patients had not proved to add major benefits in regard to immediate postoperative pain relief or reduced need for supplemental analgesics (Dahl & Møiniche, 2004; Møiniche et al., 2002b).
It was concluded as well that no overall improvement in postoperative pain control was observed after pre-emptive administration of systemic NSAID, opioids, and ketamine (Møiniche et al., 2002b). However, the addition of acetaminophen to nonsteroidal anti-inflammatory drugs (NSAIDs) within a multimodal regimen can improve pain control and reduce postoperative morphine consumption (Martinez et al., 2017). A systematic review comparing the use of NSAIDs alone or in combination with acetaminophen for postoperative pain showed that the combination was more effective than NSAIDs alone in 64% of the studies (Ong et al., 2010). The benefits of combining acetaminophen and NSAIDs, vs NSAID alone, may differ according to the procedures (Thybo et al., 2019).
Conclusions
This study come to a conclusion that the continuous infusion of MgSo4 has no added value in the control of intraoperative and the postoperative pain, as well as it has no value in the regard to decreasing the requirement for adjuvant analgesics.
Limitation of the study
The current conclusion needs to be investigated over a wider scale of patients, with an extended monitoring for the postoperative pain over a longer time frame.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request as well as on the following link MgSO4 Pre-emtive or Preventive.xlsx.
Abbreviations
- GABA:
-
Gamma-aminobutyric acid
- NMDA:
-
Non-competitive N-methyl d-aspartate
- MgSO4:
-
Magnesium sulphate
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
References
Alam A, Juurlink DN (2016) The prescription opioid epidemic: an overview for anesthesiologists. Can J Anaesth 63(1):61–68. https://doi.org/10.1007/s12630-015-0520-y
Albrecht E, Kirkham KR, Liu SS, Brull R (2013) Peri-operative intravenous administration of magnesium sulphate and postoperative pain: a meta-analysis. Anaesthesia 68(1):79–90. https://doi.org/10.1111/j.1365-2044.2012.07335.x
Buvanendran A (2011) Multimodal analgesia for perioperative pain management. Review course lectures, International Anesthesia Research Society (IARS)
Choi JC, Yoon KB, Um DJ, Kim C, Kim JS, Lee SG (2002) Intravenous magnesium sulfate administration reduces propofol infusion requirements during maintenance of propofol-N2O anesthesia: part I: comparing propofol requirements according to haemodynamic responses: part II: comparing bispectral index in control and magnesium groups. Anesthesiology 97(5):1137–1141. https://doi.org/10.1097/00000542-200211000-00017
Dahl JB, Møiniche S (2004) Pre-emptive analgesia. British Medical Bulletin The British Council 71
De Oliveira GS Jr, Castro-Alves LJ, Khan JH, McCarthy RJ (2013) Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 119(1):178–190. https://doi.org/10.1097/ALN.0b013e318297630d
Jabbour HJ, Naccache NM, Jawish RJ et al (2014) Ketamine and magnesium association reduces morphine consumption after scoliosis surgery: prospective randomised double-blind study. Acta Anaesthesiol Scand 58(5):572–579. https://doi.org/10.1111/aas.12304
Katz J, Clarke H, Seltzer Z (2011) Review article: preventive analgesia: quo vadimus? Anesth Analg 113(5):1242–1253. https://doi.org/10.1213/ANE.0b013e31822c9a59
Kelly DJ, Ahmad M, Brull SJ (2001) Preemptive analgesia I: physiological pathways and pharmacological modalities. Can J Anaesth 48(10):1000–1010. https://doi.org/10.1007/BF03016591
Ko SH, Lim HR, Kim DC, Han YJ, Choe H, Song HS (2001) Magnesium sulfate does not reduce postoperative analgesic requirements. Anesthesiology 95(3):640–646. https://doi.org/10.1097/00000542-200109000-00016
Mariano, E.R 2020. Management of acute perioperative pain, available at www.uptodate.com© UpToDate,
Martinez V, Beloeil H, Marret E, Fletcher D, Ravaud P, Trinquart L (2017) Non-opioid analgesics in adults after major surgery: systematic review with network meta-analysis of randomized trials. Br J Anaesth 118(1):22–31. https://doi.org/10.1093/bja/aew391
Mayer ML, Westbrook GL, Guthrie PB (1984) Voltage-dependent block by Mg2þ of NMDA responses in spinal cord neurones. Nature 309(5965):261–263. https://doi.org/10.1038/309261a0
McCarthy RJ, Kroin JS, Tuman KJ, Penn RD, Ivankovich AD (1998) Antinociceptive potentiation and attenuation of tolerance by intrathecal co-infusion of magnesium sulfate and morphine I rats. Anesth Analg 86(4):830–836
Møiniche S, Kehlet H, Dahl JB (2002a) A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology 96(3):725–741. https://doi.org/10.1097/00000542-200203000-00032
Møiniche S, Kehlet H, Dahl JB (2002b) A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief—the role of timing of analgesia. Anesthesiology 96(3):725–741. https://doi.org/10.1097/00000542-200203000-00032
Mudumbai SC, Oliva EM, Lewis ET, Trafton J, Posner D, Mariano ER, Stafford RS, Wagner T, Clark JD (2016) Time-to-cessation of postoperative opioids: a population-level analysis of the Veterans Affairs Health Care System. Pain Med 17(9):1732–1743. https://doi.org/10.1093/pm/pnw015
Ong CK, Seymour RA, Lirk P, Merry AF (2010) Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg 110(4):1170–1179. https://doi.org/10.1213/ANE.0b013e3181cf9281
Paech MJ, Magann EF, Doherty DA, Verity LJ, Newnham JP (2006) Does magnesium sulphate reduce the short- and long-term requirements for pain relief after caesarean delivery? A double-blind placebocontrolled trial. Am J Obstet Gynecol 194(6):1596–1602. https://doi.org/10.1016/j.ajog.2006.01.009
Rosero EB, Joshi GP (2014) Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg 134(4 Suppl 2):85S–93S. https://doi.org/10.1097/PRS.0000000000000671
Ryu JH, Kang MH, Park KS, Do SH (2008) Effects of magnesium sulphate on intraoperative anaesthetic requirements and postoperative analgesia in gynaecology patients receiving total intravenous anaesthesia. British Journal of Anaesthesia 100(3):397–403. https://doi.org/10.1093/bja/aem407
Schulz-Stubner S, Wettmann G, Reyle-Hahn SM, Rossaint R (2001) Magnesium as part of balanced general anaesthesia with propofol, remifentanil and mivacurium: a double-blind, randomized prospective study in 50 patients. Eur J Anaesthesiol 18(11):723–729. https://doi.org/10.1097/00003643-200111000-00004
Sun EC, Darnall BD, Baker LC, Mackey S (2016) Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med 176(9):1286–1293. https://doi.org/10.1001/jamainternmed.2016.3298
Suzuki H (1995) Recent topics in the management of pain: development of the concept of preemptive analgesia. Cell Transplant 4(Suppl 1):S3
Telci L, Esen F, Akcora D, Erden T, Canbolat AT, Akpir K (2002) Evaluation of effects of magnesium sulphate in reducing intraoperative anaesthetic requirements. Br J Anaesth 89(4):594–598. https://doi.org/10.1093/bja/aef238
Thybo KH, Hägi-Pedersen D, Dahl JB, Wetterslev J, Nersesjan M, Jakobsen JC, Pedersen NA, Overgaard S, Schrøder HM, Schmidt H, Bjørck JG, Skovmand K, Frederiksen R, Buus-Nielsen M, Sørensen CV, Kruuse LS, Lindholm P, Mathiesen O (2019) Effect of combination of paracetamol (acetaminophen) and ibuprofen vs either alone on patient-controlled morphine consumption in the first 24 hours after total hip arthroplasty: the PANSAID Randomized Clinical Trial. JAMA 321(6):562–571. https://doi.org/10.1001/jama.2018.22039
Wilder-Smith CH, Knopfli R, Wilder-Smith OH (1997) Perioperative magnesium infusion and postoperative pain. Acta Anaesthesiol Scand 41(8):1023–1027. https://doi.org/10.1111/j.1399-6576.1997.tb04830.x
Woolf CJ, Chong MS (1993a) Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 77(2):362–379. https://doi.org/10.1213/00000539-199377020-00026
Woolf CJ, Chong MS (1993b) Preemptive analgesia-treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 77(2):362–379. https://doi.org/10.1213/00000539-199377020-00026
Acknowledgements
We would like to acknowledge Professor Dr. Rafaat Abd Al Azzim for the wise revision and supervising of this work.
Funding
No funding source
Author information
Authors and Affiliations
Contributions
W.Y.K contributed to the data collections, idea selection, source collections, and writing. A.A.S contributed to the inclusion of source collections and revision. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Research Ethics Committee of the Faculty of Medicine (R72/2021), Ain Shams University, and conducted in accordance with the principles of the Declaration of Helsinki. A written informed consent before enrolment was taken.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamel, W.Y., Shoukry, A.A. Magnesium sulphate within multimodal analgesia, pre-emptive, or preventive analgesia. Ain-Shams J Anesthesiol 14, 7 (2022). https://doi.org/10.1186/s42077-021-00210-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-021-00210-1